This week, the U.S. joined other countries in administering the first of many COVID-19 vaccines. Pfizer’s COVID-19 vaccine’s first shipments were sent to frontline workers in all 50 states. This shipment followed a green light from the U.S. Food and Drug Administration. Moderna’s vaccine is still awaiting approval but expects to follow Pfizer with shipments next week.
Continue reading below for an overview of current cases, how vaccines are being doled out, and an introduction to a new rapid at-home COVID-19 test.
Present Cases and Future Projections
The vaccines bring much-needed hope and optimism as the country’s death toll pushes past 300,000. With the holiday season quickly approaching and groups gathering to celebrate despite caution, officials are concerned about an influx in cases and deaths. Some states are extending COVID-19 restrictions in light of this. Mexico is extending its restrictions on U.S. travelers through January 21.
In total, Pfizer will distribute 2.9 million vaccine doses this week. With a population of more than 328 million and two doses of vaccine needed, it will be some time before case numbers and deaths decrease and even longer before full immunity is met. With this understanding, the recommendation is that everyone continues to wear masks and social distance.
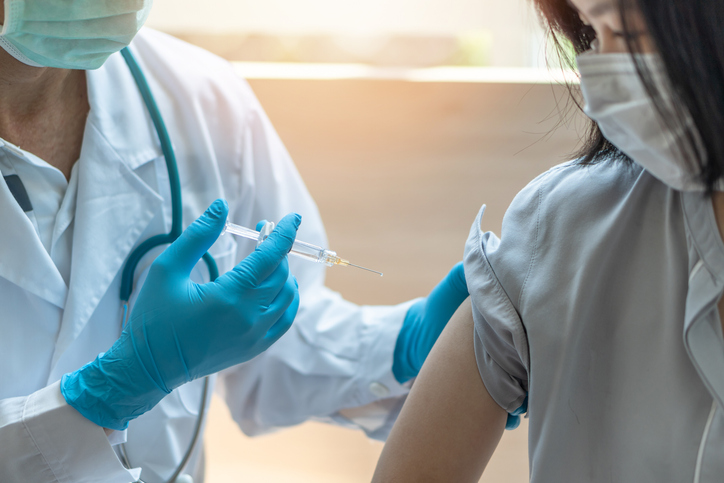
Vaccine Administration
At present, the vaccines are only available to those who are above the age of 16. While state government officials have the power to decide who receives the first shipments, the CDC has recommended they go to healthcare professionals and the elderly, specifically those in nursing homes. Many nursing homes are set to receive vaccines next week through the Federal Pharmacy for Long-term Care Program.
Side effects of the COVID-19 vaccine are similar to those of others. Individuals who receive the shot may experience pain at the injection site and a slight fever over the next day or two. Only those who have had severe allergic reactions to one of the vaccine ingredients are advised not to take it. Still, those with compromised immune systems and ongoing health issues should consult their physicians before getting the vaccine.
At-Home Testing
In the meantime, the FDA just approved an at-home COVID-19 test. This test will be available over-the-counter and doesn’t require the sample to be sent to a lab. Those who suspect they may have the virus will know within 20 minutes of taking the test.
This test’s speed and convenience may limit the spread of the virus that occurs between test administration and the provisioning of results. The test shows promise in lowering COVID-19 case numbers, correctly identifying over 90% of positive samples in individuals with and without trademark symptoms.
Want to rotate in the safety and comfort of your own home?






Leave A Comment